NEES News
The Proof Is in the Pores
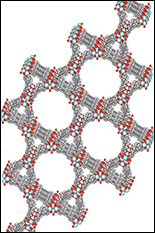
This article is reprinted with permission from "Frontiers in Energy Research", 2015 Spring edition, a newsletter of the Department of Energy. If molecules were Lego blocks, then metal-organic frameworks (MOFs) would be Lego castles; highly ordered three-dimensional structures resulting from linking molecular building blocks. These porous materials have attracted scientists for over a decade due to their application in gas storage, chemical sensors, and catalysis. With improvements in computational design and new experimental approaches, MOFs are increasingly being applied to energy science as well as electronics and optical switching. The high surface area of MOFs is what makes these materials so unique, offering a large interfacial area for interactions between MOF walls and guest molecules within the pores. This property is being investigated at several Energy Frontier Research Centers for clean energy technologies. At the Center for Gas Separations Relevant to Clean Energy Technologies (CGS), MOFs with ultrahigh porosity are being developed for processes that rely on selective adsorption of gases. Initially, MOFs were developed for carbon dioxide sequestration, but now the CGS efforts have extended to other compounds of interest, including hydrocarbons and hydrogen. The separation and storage of hydrogen (H2) using MOF materials offers a viable route for capturing hydrogen in photoelectrochemical cells, which use the energy from sunlight to split water into oxygen and hydrogen. Hydrogen-capturing MOFs could therefore play an important role in solar fuel technology. Researchers at CGS are also working to engineer more robust frameworks that can withstand harsh conditions without breaking apart. For example, researchers recently reported a unique zirconium-porphyrin MOF (PCN-223) with an unusual disordered arrangement that was prepared by assembling molecular subunits under kinetic control. The extremely high connectivity in PCN-223 imparted exceptional chemical stability. The MOF was stable in aqueous environments with a pH ranging from 0 to 10. The framework was also an effective catalyst for the hetero-Diels-Alder reaction and could easily be recycled from the reaction medium by simply filtering off the MOF catalyst. Recyclable MOF catalysts are important for green chemistry, as they improve the overall energy efficiency and reduce waste. With an ever-growing number of molecular building blocks that can be used to make MOFs, one of the current challenges is rational material design: how can we predict the chemical and physical properties of MOF structures? At the recently established Inorganometallic Catalyst Design Center(ICDC), researchers are turning to computational simulations to understand structure-property relationships in MOFs. The ICDC brings together a diverse group of scientists with expertise in computational modelling of electronics and reactivity of MOFs, as well as the synthesis and characterization of new MOF materials. A major thrust of the research carried out at the ICDC is directed towards establishing a better fundamental understanding of structure-property correlations in MOF materials to guide the synthesis of new MOFs with a desired catalytic function. In addition to the more conventional applications of gas storage and catalysis, MOFs have received recent attention as materials for electronic and photonic applications. The exceptional ability to control the solid-state arrangement of molecular subunits within MOFs has enabled a new paradigm for the construction of materials with complex optoelectronic functions. For example, in a recent study carried out at the CGS, a MOF that exhibited reversible control of singlet oxygen generation was constructed by employing a multifaceted structure incorporating a photochromic (light-responsive switch) subunit and a singlet oxygen sensitizer subunit. Using this MOF, the generation of singlet oxygen is under remote control, switched on and off by simply shining light of different wavelengths on the MOF. Singlet oxygen can provoke reactions to treat industrial wastewater, sterilize blood, and eliminate cancerous cells, undesired herbs or pests. Moreover, the ability to recapture and reuse the singlet-oxygen-sensitizing MOF is attractive from a sustainability perspective. At the [UMD-led] Center for Nanostructures for Electrical Energy Storage (NEES) and the Center for Excitonics(CE), research groups are taking new approaches to two-dimensional electronic and excitonic materials based on MOFs. One of the major thrusts at the CE is to better understand how the optical properties of MOFs are affected by the molecular building blocks and their structural organization within the framework. Exciting new properties have been discovered, including coordination-induced emission and high-temperature fluorescence in some MOFs. Recent work carried out at the CE showed that the unique high-temperature fluorescence observed in MOFs containing tetrakis(4-carboxyphenyl)ethylene ligands can be used to improve the selectivity of optical sensors and demonstrated ammonia-selective sensing at 100 °C. The straightforward device fabrication of MOF-based sensors offers the potential for energy-efficient manufacturing of devices with applications in defense, food packaging, and environmental monitoring. Also, MOFs can act as electrical sensors, as recently demonstrated at the NEES by Alec Talin and co-workers. Here, the team discovered a new MOF with tunable conductivity spanning six orders of magnitude. The changes in conductivity were brought on by a transformation of the MOF's electronic structure after infiltration of its pores with an electron-accepting guest molecule. The observation of tunable conductivity in this framework brings MOFs into a new spotlight, opening doors for previously unexplored applications in electronics. For the many applications of MOF materials a common theme resounds: it's all about the interface! Whether it's the interaction between a MOF and an adsorbed gas molecule, or an organic compound undergoing a catalytic transformation, the functional surfaces of MOFs are what impart such exciting properties to these materials. The unique ability to tune both the functional molecular components and the crystalline geometric structure of MOFs has had a significant impact on a wide range of applications. The chemistry that occurs inside the pores of MOFs is as diverse as the applications of these materials, making MOFs an important piece of the energy puzzle. --end-- About the author: Hayden T. Black is a postdoctoral fellow in the Department of Chemistry at the Georgia Institute for Technology, supervised by John R. Reynolds. He is a member of the Center for Solar Fuels (UNC), an Energy Frontier Research Center. He received his Ph.D. from the University of North Carolina at Chapel Hill in 2012 where he synthesized and studied organic semiconductors for transistor applications. He went on to carry out a postdoctoral appointment at McGill University, working with Dmitrii F. Perepichka on the supramolecular assembly of organic semiconductors via hydrogen bonding. His research now focuses on the synthesis of organic chromophores and organic semiconductors for applications in photovoltaics and photoelectrochemical cells.
Acknowledgments:
Talin et al.: First author A.A.T. was supported by the Center for Nanostructures for Electrical Energy Storage (NEES), an Energy Frontier Research Center funded by the DOE, Office of Science, Office of Basic Energy Sciences. This work was also supported by the Laboratory Directed Research and Development Program at Sandia National Laboratories and the U.S. Department of Energy (DOE) SunShot Program, which grew the MOF films and performed structural and chemical characterization. F.E.G. was supported by the DOE, Office of Science, Office of Basic Energy Sciences, Division of Materials and Engineering Sciences. A.C., R.A.K., and H.P.Y. acknowledge support under the Cooperative Research Agreement between the University of Maryland and the National Institute of Standards and Technology Center for Nanoscale Science and Technology through the University of Maryland. For more information on this work, see the article in this issue.
More Information:
Feng D, ZY Gu, YP Chen, J Park, Z Wei, Y Sun, M Bosch, S Yuan, and HC Zhou. 2014. "A Highly Stable Porphyrinic Zirconium Metal-Organic Framework with shp-a Topology." Journal of the American Chemical Society 136(51):17714-17717. DOI: 10.1021/ja510525s Park J, D Feng, S Yuan, and CH Zhou. 2015. "Photochromic Metal–Organic Frameworks: Reversible Control of Singlet Oxygen Generation." Angewandte Chemie International Edition 54, 430-435. DOI:10.1002/anie.201408862 Shustova NB, AF Cozzolino, S Reineke, M Baldo, and M Dinca. 2013. "Selective Turn-On Ammonia Sensing Enabled by High-Temperature Fluorescence in Metal–Organic Frameworks with Open Metal Sites." Journal of the American Chemical Society 135(36):13326-13329. DOI: 10.1021/ja407778a Talin AA, A Centrone, AC Ford, ME Foster, V Stavila, P Haney, RA Kinney, V Szalai, F El Gabaly, HP Yoon, F Léonard, and MD Allendorf. 2014. "Tunable Electrical Conductivity in Metal-Organic Framework Thin-Film Devices." Science 343(6166):66-69. DOI: 10.1126/science.1246738
March 12, 2015 Prev Next |