NEES News
"Guests" of Honor
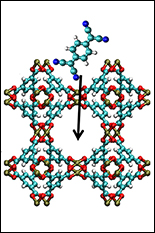
This article is reprinted with permission from "Frontiers in Energy Research", 2015 Spring edition, a newsletter of the Department of Energy. A solar panel sits in the sun to absorb photons and process them into electricity. As the sun's rays strike the dark surface, the solar panel gets hot and energy is wasted as it is lost as heat. Car engines, gas pipelines, and even computers waste energy through heat, so researchers have been looking for ways to capture this waste heat and improve efficiency. Researchers at the [UMD-led] Nanostructures for Electrical Energy Storage (NEES) Energy Frontier Research Center recently discovered metal-organic frameworks (MOFs) with tunable electrical conductivity, and these exciting new materials could open up applications in waste heat capture, as well as sensors and flexible electronic devices. MOFs are crystalline materials made from metal ions linked by organic molecules that form nano-sized pores (see "The Proof Is in the Pores"). MOFs are usually poor conductors because the organic or carbon-rich "linker" molecules make the overall material electrically insulating. Researchers at NEES first described MOFs with tunable conductivity in early 2014 in an article in Science. Before this work, only a few MOFs were electrically conductive, relying on a very specific framework. These new materials are not only more conductive, their conductivity can be tuned across six orders of magnitude. Special Guests Check into the Pores.To create tunable, conductive MOFs, the researchers at NEES had to think outside the box—or rather, inside the pores. Rather than designing the framework to carry a current on its own, they built a device where guest molecules inside the pores transport electrical charge. To realize this experimentally, they first synthesized a thin film (100 nanometers thick, about 1,000 times thinner than a piece of paper) of a copper-based MOF on a silicon wafer. They removed the water that had been trapped in the pores during synthesis and plunged the wafers into a solution of TCNQ, a molecule with unique charge-transfer properties. After 72 hours, the thin film was saturated with TCNQ. The conductivity of the thin film increased from ~10-6 Siemens/meter (units of electrical conductivity) before the TCNQ incorporation to a maximum conductivity of 7 Siemens/meter. The fully infiltrated MOF is about as conductive as seawater—1 million times more conductive than the unmodified material. The conductivity of these MOFs can be tuned by varying the exposure time to TCNQ. More exposure leads to higher TCNQ concentrations in the pores and higher conductivity. The Theory behind the Conductivity. Metals are conductive because metal atoms don't hold tightly to their valence electrons (electrons in the outer shell of each atom). Because these electrons don't stick to just one local atom, they are said to be delocalized, and they flow freely from atom to atom in the presence of an electric field, conducting electricity. In organic molecules, however, each atom typically holds tightly to these valence electrons, which cannot flow. This is why plastics are often used as insulation for wires. However, a special class of organic molecules can conduct electricity. What makes these conductive organics different is that they possess a network of delocalized electrons, a special bridge that enables electrons to flow freely through the otherwise insulating material. TCNQ is one of these special conductive organic molecules. Computer simulations of the MOF framework and the TCNQ revealed how conductivity arises in these materials. After entering the pores, the TCNQ molecules line up end-to-end with the copper ion clusters in the framework. As the delocalized electrons in TCNQ interact with those in the copper, they form conducting bridges throughout the material. Reducing the concentration of TCNQ, or swapping out TCNQ for other molecules with poorly matched electronics, reduced the conductivity, verifying the theoretical predictions. Understanding the underlying mechanism behind this adaptable conductivity will be important for developing new materials that work by the same principles. Future Research Directions. This discovery marks the demonstration of tunable, conductive, nanoporous materials with the crystalline order of MOFs—a new class of materials that could enable applications such as flexible electronic devices, reconfigurable electronics, sensors, and low-cost thermoelectric devices for converting waste heat to energy. Looking forward to future discoveries, Talin said, "Our discovery represents a new class of materials we call Guest@MOFs with unique properties stemming from the strong interactions between the MOF and different guests. By developing a deeper understanding of these interactions, we hope to discover new Guest@MOF materials with properties optimized for a variety of applications." The researchers discuss this new concept in a perspective article to soon appear on the cover of Journal of Physical Chemistry Letters. --end-- About the author: Tyler Josephson is a Ph.D. candidate at the University of Delaware and is a student in the Catalysis Center for Energy Innovation. He is advised by Dion Vlachos. He is using computational tools to fundamentally understand solvent effects in reactions used to produce fuels and chemicals from biomass. Tyler holds a B.S. in chemical engineering from the University of Minnesota.Acknowledgments: This work was supported by the Laboratory Directed Research and Development Program at Sandia National Laboratories and the U.S. Department of Energy (DOE) SunShot Program, which grew the MOF films and performed structural and chemical characterization. First author AAT was supported by Nanostructures for Electrical Energy Storage (NEES), an Energy Frontier Research Center funded by the DOE, Office of Science, Office of Basic Energy Sciences. FEG was supported by the DOE, Office of Science, Office of Basic Energy Sciences, Division of Materials and Engineering Sciences. AC, RAK, and HPY acknowledge support under the Cooperative Research Agreement between the University of Maryland and the National Institute of Standards and Technology Center for Nanoscale Science and Technology through the University of Maryland. More Information: Stavila, V, AA Talin, and MD Allendorf. 2014. "MOF-Based Electronic and Opto-Electronic Devices."Chemical Society Reviews 43:5994-6010. DOI: 10.1039/c4cs00096j Talin AA, A Centrone, AC Ford, ME Foster, V Stavila, P Haney, RA Kinney, V Szalai, F El Gabaly, HP Yoon, F Léonard, and MD Allendorf. 2014. "Tunable Electrical Conductivity in Metal-Organic Framework Thin-Film Devices." Science 343(6166):66-69. DOI: 10.1126/science.1246738
March 12, 2015 Prev Next |